Introduction
The Coronavirus Disease 2019 (COVID-19) outbreak caused a public health emergency across the world. The emergent situation caused a threat to both ongoing and new clinical trials, especially at the beginning of the pandemic in early 2020. In particular, the COVID-19 pandemic impacted participants’ ability to come to planned clinic visits due to site closures, travel restrictions, illness, and disrupted dosing within clinical trials. During the pandemic, clinical trials’ protocols and procedures evolved rapidly, supported by a variety of technologies, including phone contact, virtual visits, and alternative locations for assessments to provide accessibility of clinical trials’ visits for participants.
For these reasons, trial disruptions as well as missed, late, and remote visits were common, as was the need to document confirmed COVID-19 diagnoses in participants. In some cases, standards existing pre-pandemic were difficult to capture due to such temporally irregular data.
At the same time, regulatory guidance was prompt with recommendations for handling discontinuities caused by the pandemic. In early 2020, the World Health Organization (WHO) published the COVID-19 Case Definitions1 and the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) published the Guidance for Industry/Points to Consider document for implications of COVID-19 pandemic for ongoing clinical trials.3, 4, 5 The FDA acknowledged pandemic-induced difficulties in meeting administering or using the investigational product and adhering to protocol-mandated visits and testing. The FDA issued guidance and general considerations to “assist sponsors in assuring the safety of trial participants, maintaining compliance with good clinical practice (GCP), and minimizing risks to trial integrity” during the COVID-19 public health emergency.3
Before the COVID-19 pandemic, there were no industry standards to capture and assess pandemic study impacts. The Clinical Data Interchange Standards Consortium (CDISC) developed and published the interim therapeutic area user guide (TAUG) for COVID-19 in April 2020,2 which was used as a reference to develop and implement standards by clinical trial sponsors. A cross-functional GlaxoSmithKline (GSK) team was formed to assess COVID-19 pandemic impacts to clinical trials and the applicability of newly developed, relevant standards and regulatory requirements.
Methods
A cross-functional team within GSK consisting of representatives from Clinical, Biostats, Data Management, Data Standards, electronic case report form (eCRF) Designers, and Safety worked together to develop and implement end-to-end standards for COVID-19 pandemic impacts in early 2020.
The team referred to WHO COVID-19 Case Definitions,1 CDISC COVID-19 Interim TAUG,2 and FDA and EMA published Guidance for Industry/Points to Consider documents for COVID-19 pandemic impacts for ongoing clinical trials.3, 4, 5 Although we consulted CDISC Standards, including the Guidance for Disrupted Studies,2 there was an urgent need to fill in the gaps for the components of GSK end-to-end standards not covered by CDISC. Therefore, we referred to the available industry standards/guidance, including the draft shared for public review, determined the standards to be developed, and developed GSK’s internal standards to be fit-for-purpose. The standards contain eCRF, study data tabulation model (SDTM)/Analysis Data Model (ADaM) dataset specifications, display standards, and standard macro/programs for their creation.
Per regulatory and industry guidelines, revisions were made after implementation as details were provided. Discussions are ongoing and further revisions of the standards may be required considering the evolving COVID-19 situation.
Results
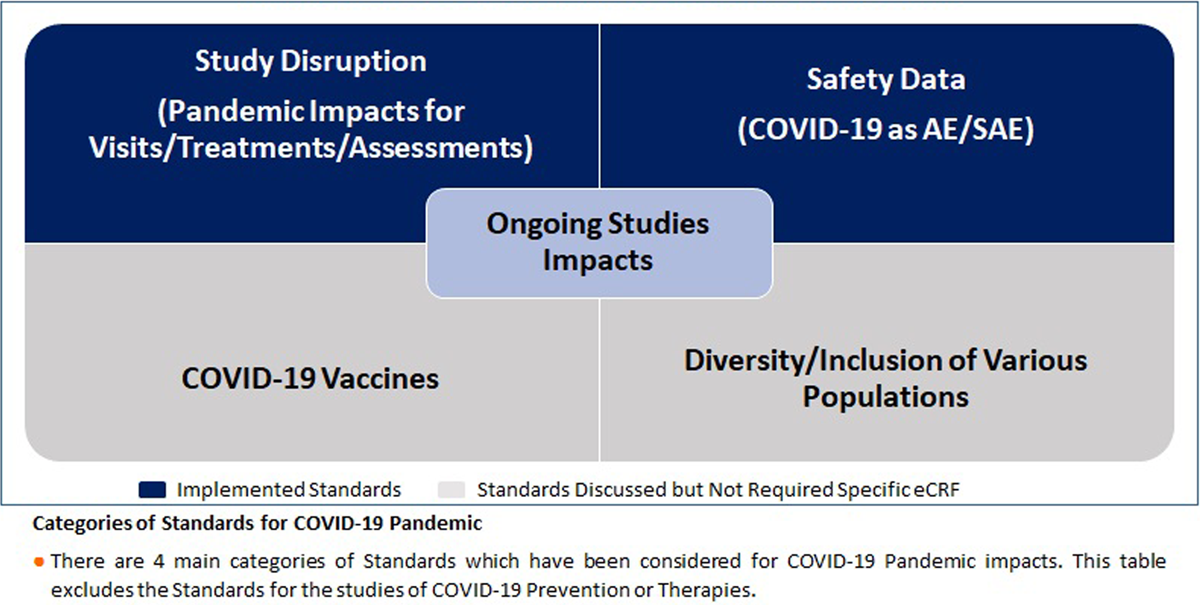
We classified the standards for the COVID-19 pandemic into four categories: 1. Safety data (COVID-19 reported as Adverse Event (AE)/Serious Adverse Event (SAE)), 2. Study disruption, 3. COVID-19 vaccines, 4. Diversity/inclusion of various populations (Table 1). Our standards for COVID-19 pandemic impacts allowed us to effectively capture and report the pandemic impacts to the clinical trials.
Implemented standards
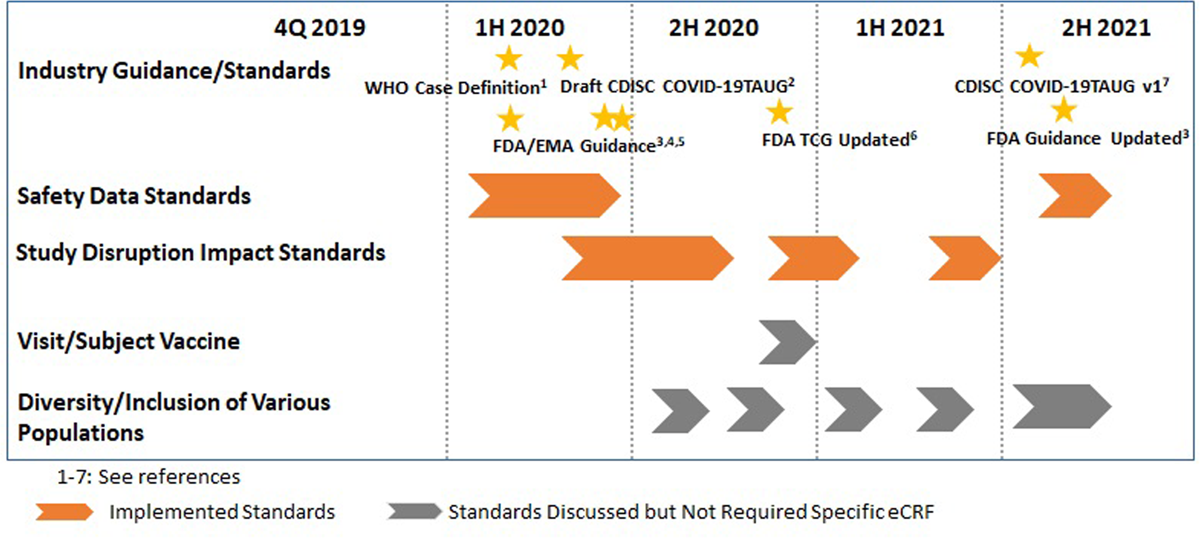
Two of four categories of COVID-19 pandemic impact standards were developed and implemented for all ongoing and new clinical trials in 2020 (Figure 1). The implementation of the additional standards was done for all ongoing and new studies within four weeks after creation of the standards.
1. Safety data (COVID-19 as AE/SAE)
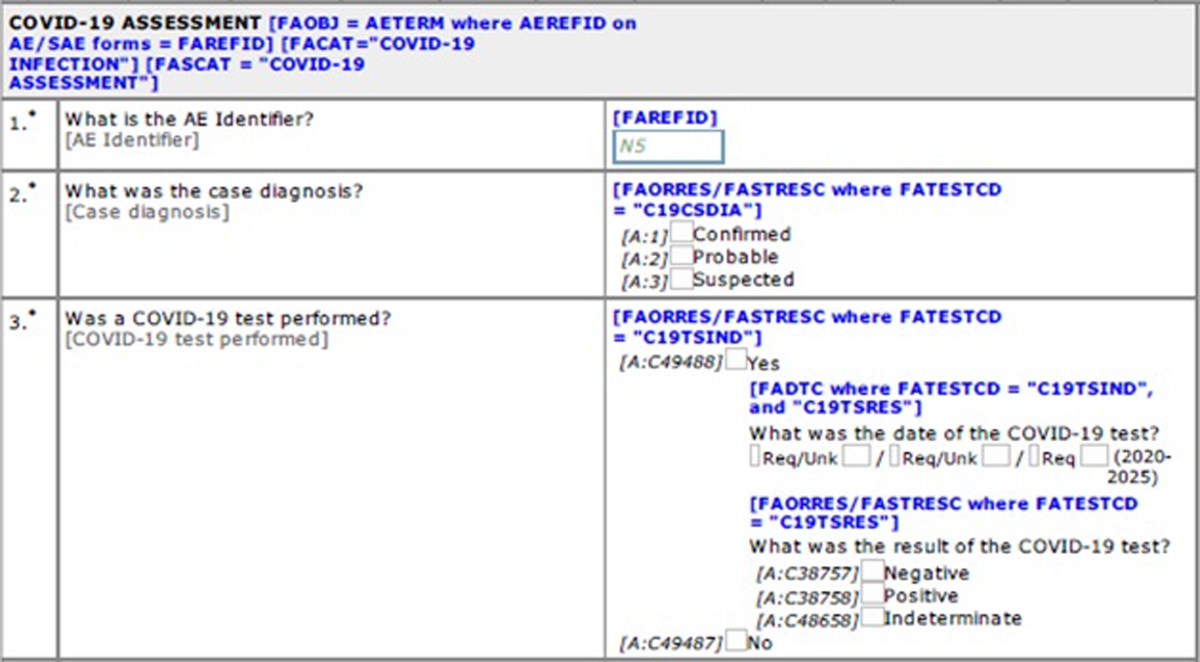
COVID-19 infection or infection-related adverse events were captured using the existing standards for AE/SAE. A new standard eCRF was developed to capture additional supportive data (i.e., WHO Case Definition (Suspected case, Probable case, Confirmed case))1 and summary results of COVID-19 tests (Figure 2). SDTM standards, ADaM standards, and display standards were also developed and implemented. There were multiple options for SDTM mappings for these additional data, but we decided to map additional data to the Findings About Adverse Events (FAAE) dataset, as we are capturing high level findings for COVID-19 reported as AE/SAE.
In late 2021, it was recognized that due to the broad community transmission of COVID-19 and COVID-19 lab testing being widely available, some of the data captured at the beginning of the pandemic in the new standard eCRF was redundant. As such, the standard was updated to capture the WHO case definition only, along with the usual AE/SAE information. The simplified standard is being applied to all new studies.
2. Study disruption (pandemic Impacts for visits/treatments/assessments)
A standard eCRF was developed and implemented for all studies to produce standard statistical outputs that quantify the impact of the pandemic on study visits, study treatments, and study withdrawals.
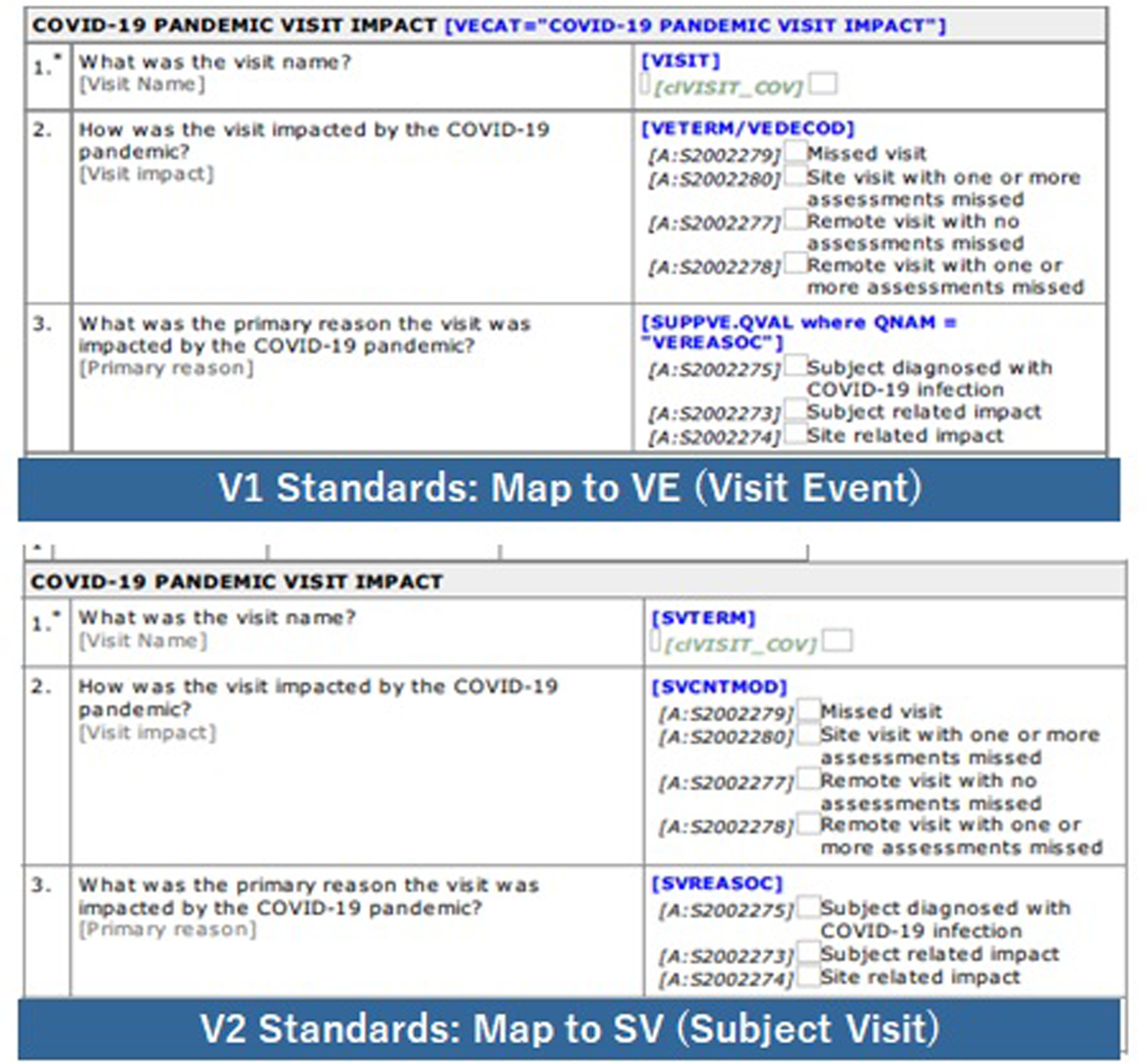
In terms of the impacts for patient visits, we initially considered using protocol deviations (DV) and domain in SDTM. However, a custom event class SDTM domain, visit events (VE) was instead implemented, as it aligned with the original example in the CDISC COVID-19 Interim TAUG.2 About a year after the initial implementation, we changed the standard SDTM mapping from VE to subject visits (SV) considering updated guidance from the US FDA Study Data Technical Conformance Guide6 and upcoming CDISC SDTM version (Figure 3).
Evolution of eCRE for Visit Impact.
Note: Initial release of the Visit Impact standards was VE (Visit Event) to store Pandemic Visit impact and to align with the CDISC COVID-19 Interim TAUG.2
We changed standard SDTM mapping from VE to SV (Subject Visits) considering the US FDA Study Data Technical Conformance Guide.6
In late 2021, we further enhanced the capture of this information. We made changes so that the reason for missed visits and assessments could be captured as part of standard functionality with the EDC system. In case the visit/assessment is impacted due to the COVID-19 pandemic, the data are mapped to the SDTM SV domain and contribute to produce the same ADaM dataset/display standards.
Standards discussed but not changed
In addition to the implemented standards, two other categories of standards were discussed, but it was decided no specific changes to eCRFs or other standards were required, as the global level standards at this point and time were sufficient (Figure 1).
3. COVID-19 vaccines
Because COVID-19 vaccines have been developed and distributed rapidly across the world during the pandemic, it was recognized that many study participants would have the COVID-19 vaccine administered prior to or during a clinical study. We determined that capturing COVID-19 vaccine use as a part of standard concomitant medications (CM) eCRF was sufficient for most of our studies. For a subset of studies where lymphadenopathy may be of particular interest, a new standard was created to capture more detailed information around the COVID-19 vaccine, including vaccine type, dose number, and anatomical location of injection site, which can be stored as standard or non-standard variables for the studies.7
4. Diversity/inclusion of various populations
There is both ongoing work in our organization to ensure diversity and inclusion of underrepresented populations in the COVID-19 clinical trials and consideration of whether additional data points are required to assess diversity. The CDISC HIV TAUG served as a resource for these discussions.8
Discussion
Due to the COVID-19 pandemic, we were challenged by stakeholders (including regulatory authorities) to collect new data to support the assessment of the impact of the pandemic on ongoing clinical trials. There were no standards for these new data, although regulators provided guidance and CDISC produced an interim user guide.2, 3, 4, 5 We used these to develop new internal standards to collect these new data. In developing GSK standards, we tried to minimize the operational impact to sites and focused on the minimum viable product for clinical research reporting needs. We implemented these as quickly as possible to capture the data in a timely manner. However, implementation required additional eCRFs/additional data points and post go-live changes to the EDC system used for ongoing clinical trials.
The pandemic has accelerated the evolution and adoption of new technologies and methodologies in how clinical trials are being conducted. Before the pandemic, solutions to support decentralized clinical trials were available, yet they were not routinely utilized in our studies. The pandemic meant that solutions for home nursing and remote visits were implemented in many of our studies. These changes supported sites and patients who were experiencing disruptions in terms of ability to travel to clinical trial sites and/or an ability and willingness to do so during country-level lockdowns. It also meant we minimized, where possible, visits and assessments being missed.
This could potentially expand the landscape of sources for clinical trial data, including electronic health records, eDiary, device data, remote visits, remote lab data, and so forth. Currently, the industry standards for these data are not fully available. When industry standards are available for these different varieties of source data for our clinical trials’ databases, the number of additional data points manually entered into EDC systems and the number of post go-live eCRF changes needed will be reduced while increasing the accuracy of the data and improving the efficiency of the clinical trials.
Conclusion
The cross-functional team successfully developed and implemented end-to-end standards for COVID-19 pandemic impacts and at pace in response to the emerging pandemic in early 2020. This enabled the capture and assessment of the pandemic impacts to the ongoing clinical trials in a standard way with minimum site burden and minimal disruption to protocols and internal standards. As the pandemic evolved, we monitored the impact on our standards so that any required revisions could be identified and made in a timely fashion. This is an ongoing process, with further revisions to the standards likely required as the COVID-19 pandemic evolves, ensuring that we continue to capture fit-for-purpose data as required per regulatory guidelines. The presence of the CDISC guidance on disrupted studies2 and the collaboration of a cross-functional team were key to adjusting the standards and rapidly implementing the changes. This experience provided a process for responding to future pandemics or other societal disruptions.
Acknowledgements
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the following individuals for their contributions and critical review during the development of this manuscript: Mayank Anand, Mike Olds.
Competing Interests
The authors have no competing interests to declare.
References
World Health Organization. COVID-19: Case Definitions. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2. Published March 2020, revised in December 2020. Accessed March 20, 2020.
Clinical Data Interchange Standards Consortium, Inc. Guidance for Ongoing Studies Disrupted by COVID-19 Pandemic Version 1.0. https://www.cdisc.org/system/files/members/standard/ta/Guidance_for_Ongoing_Studies_Disrupted_by_COVID-19.pdf. Accessed April 21, 2020.
U.S. Department of Health and Human Services Food and Drug Administration. Conduct of Clinical Trials of Medical Products During the COVID-19 Public Health Emergency, Guidance for Industry, Investigators, and Institutional Review Boards. FDA-2020-D-1106-0002. https://www.fda.gov/media/136238/download. Published March 2020, revised in August 2021. Accessed September 13, 2021.
U.S. Department of Health and Human Services Food and Drug Administration. Statistical Considerations for Clinical Trials During the COVID-19 Public Health Emergency Guidance for Industry. FDA-2020-D-1136. https://www.fda.gov/media/139145/download. Published June 2020. Accessed September 13, 2021.
European Medicines Agency. Points to consider on implications of Coronavirus disease (COVID-19) on methodological aspects of ongoing clinical trials. EMA/158330/2020. https://www.ema.europa.eu/en/documents/scientific-guideline/points-consider-implications-coronavirus-disease-covid-19-methodological-aspects-ongoing-clinical_en-0.pdf. Published June 29, 2020. Accessed September 13, 2021.
U.S. Department of Health and Human Services Food and Drug Administration. Study Data Technical Conformance Guide, Technical Specifications Document. https://www.fda.gov/media/153632/download. Published March 2022. Accessed September 13, 2021.
Clinical Data Interchange Standards Consortium, Inc. Vaccine Administration v1.0 (Final), Version 1.0. https://www.cdisc.org/system/files/members/standard/ta/Vaccine_Administration%20_v1.0_Final_0.pdf. Published June 22, 2021. Accessed September 13, 2021.
Clinical Data Interchange Standards Consortium, Inc. Therapeutic Area User Guide for HIV, Version 1.0. https://www.cdisc.org/system/files/members/standard/ta/HIV/TAUG-HIVv1.0_2019-01-18.pdf. Published January 18, 2019. Accessed September 13, 2021.