Introduction
Electronic Data Capture (EDC) study designers can benefit from the adoption of high-quality content and study design standards. This report describes the implementation of validated case report form (CRF) content using the Clinical Data Acquisition Standards Harmonization (CDASH)1 obtained from the Clinical Data Interchange Standards Consortium (CDISC) eCRF Portal ( https://www.cdisc.org/kb/ecrf) within an eCRF content library in the OpenClinica EDC system,2 including considerations for field mapping, naming conventions, and dissemination.
CDASH forms that are tested, curated, and easily accessible within the EDC system can reduce barriers to their effective use, both in studies conducted for regulatory submission/approval (the primary audience for which CDASH was designed) and other “non-regulatory” study types. Study designers, builders, and data managers have the potential to benefit from improved quality and saved time by using these pre-built, expert-led CRF definitions. The objective of this report is to demonstrate technical feasibility and to produce high-quality, standards-based electronic Case Report Form (eCRF) templates that study designers can easily adopt from a centralized library. Belief will increase adoption of CDASH standards amongst study designers, ultimately improving overall design efficiency and study quality.
Clinical research professionals usually think of there being significant distinctions between two categories of clinical research: studies conducted for regulatory submission/approval and studies not intended for that purpose.8 While it is true there are many differences across these types of study, there are also many similarities in requirements, design, and execution. One core similarity is that all studies benefit from use of well-designed eCRFs with appropriate data definitions and controlled terminologies, built on input from scientific, statistical, and operational subject matter experts. Research conducted by academic, non-profit, and public health organizations is often of the “non-regulatory” type, and may use observational and interventional, prospective, and retrospective study designs. Despite the diversity of these types of studies, their eCRFs have common purposes, such as to collect participant demographics, medical histories, concomitant medications, adverse events, and therapeutic area-specific information. However, there is frequently a lack of standardization and re-use of eCRF content across organizations or even across studies, including in data element naming conventions, question text, question order, controlled terminologies, and overall design. The CDASH library was created to help to address this lack of standardization.3 CDASH establishes a uniform way to collect data across studies and across organizations. Originally developed for use in regulatory submissions, the CDASH standard can be used by any organization or individual involved in the collection, preparation, and analysis of clinical research data, and can add value in standardizing and raising eCRF quality for academic, non-profit, and public health research. The CDASH eCRFs establish “a standard way to collect data consistently across studies and sponsors so that data collection formats and structures provide clear traceability of submission data into the Study Data Tabulation Model (SDTM), delivering more transparency to regulators and others who conduct data review”.1
OpenClinica is an EDC system used in both trials for regulatory submissions by industry and in academic research.2 It combines regulatory compliance features (Good Clinical Practice, Computer Systems Validation, 21 CFR Part 11) for randomized interventional trials with flexibility for diverse study designs. It allows the design and implementation of eCRF content by non-technical users, and features portability of eCRF definitions for reuse across studies and organizations. Among other capabilities, OpenClinica includes a library management system that enables sharing, discovery, and reuse of CRF and data element content. OpenClinica is built to facilitate and support data standards, with a data model and APIs designed around the CDISC Operational Data Model (ODM) standard.4
To help clinical and translational researchers with data collection, storage, and transfer, CDISC and OpenClinica have collaborated to demonstrate implementation of the CDASH eCRF metadata in an EDC form library and subsequent use and reuse of the standard forms in study builds. The eCRFs are targeted towards use in the design of trials for regulatory submissions by industry, which is the traditional audience for CDASH, and towards academic researchers.
Methods
The CDISC eCRF Portal offers CDASH form content in PDF, HTML and XML formats, to use as is or to import to an EDC system for customization.5
CRFs in OpenClinica are defined using the XLSForm specification, a community standard used to help simplify the authoring of forms in Excel, originally developed by Andrew Marder and Alex Dorey of the Sustainable Engineering Lab at Columbia University.6 XLSForm is a “practical standard for sharing and collaborating on authoring forms”.6 The specification is easy to apply to simple forms for easy start up, but also supports the authoring of complex forms.6 After an XLSForm is defined and published to an OpenClinica study, it is represented in CDISC ODM XML. To implement the CDASH domains in OpenClinica, a crosswalk between CDASH, ODM, and XLSForm was required. We performed analysis of these three models and created an alignment of the major components of each, depicted in Table 1. The alignment takes ODM-XLSForm mappings already implemented in the EDC software into account.
CDASH to ODM to XLSForm Mappings, with key implementation details for XLSForm eCRF template.
| CDASH Element | ODM Element/Attribute | XLSForm | Key Implementation Details |
|---|---|---|---|
| Forms.Name | FormDef.Name | OpenClinica Form Name | n/a |
| Forms.OID | FormDef.OID | Derived from OpenClinica Form Name | n/a |
| Section.Domain | ItemGroupDef.OID | survey.bind::oc:itemgroup | Create a group for every item group defined in the CDASH CRFs, begin/end group for non-repeating item groups, begin/end repeat for repeating item groups using the same as the bind::oc:itemgroup value as used for items in the item group. |
| Questions.Name | ItemDef.Name | survey.name | n/a |
| Questions.Aliases.prompt | ItemDef.OID | Derived from survey.name | ItemDef.Name with parentheses, gets translated to be the OID, so be prescriptive and match CDASH standards. |
| Question.Text | ItemDef.Question | survey.label | ItemDef.Question.Translated text with no lang if present, otherwise ItemDef.Question.TranslatedText where lang=en. Line breaks removed and multiple spaces compressed to a single space. |
| Data Type | ItemDef.DataType | survey.type | For items: If ItemDef.CodeListOID is not missing, select_multiple if ItemDef alias (where Context=completionInstructions) contains “Check all that apply”; otherwise select_one. Added list_name in both cases. Otherwise, ItemDef.DataType replaces “float” with “decimal” and “time” with “text” (see constraint). |
| Terminologies.Name | CodeList.Name | choices.list_name | CodeListDef.OID with .()-/ replaced with spaces, then repeated spaces compressed to a single space and spaces replaced with underscores. This is used for reference of the codelist in the survey tab. |
Naming Conventions
Within this mapping, shown in Table 1, several conventions were created for populating the eCRF definitions, to align with CDASH standards and to ensure reasonable user experience within the EDC implementation:
Item OIDs: OIDs are the unique identifiers of objects within OpenClinica. At the item/question level, OIDs are generated by the system based on the item name (survey.name) provided in the form definition. OIDs are required to be completely unique for the entire study (across all CRFs used in a study). To avoid potential conflicts, the domain is added as a prefix for each OID, eg VS_HR for Heart Rate in the Vital Signs domain.
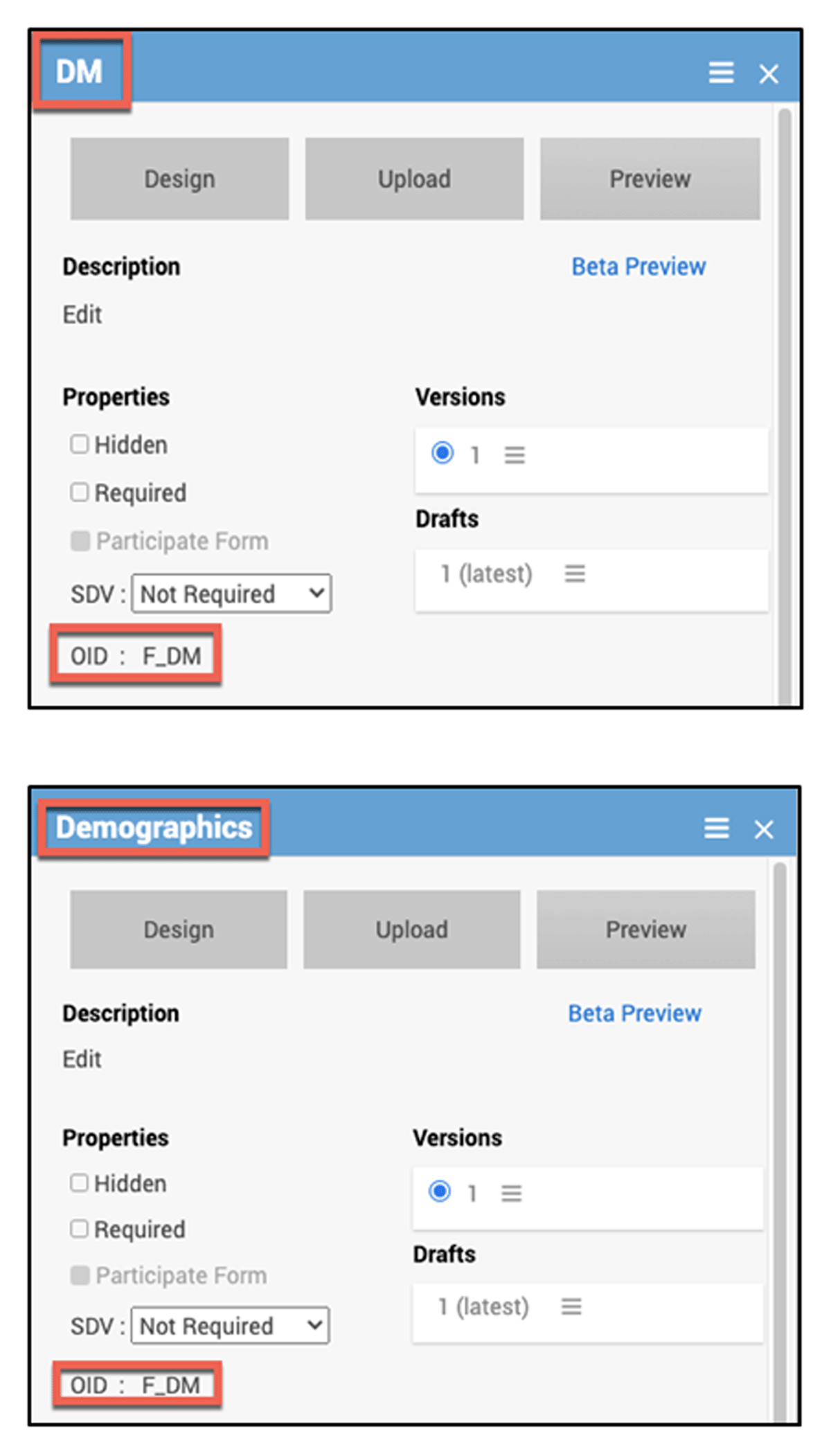
Form OIDs: Form OIDs are auto-generated upon initial naming of the form. To make sure that the OID matches the CDASH Domain, the form was initially created using the Domain as the name of the CRF when first adding the form, as the Form OID is generated from this name. After the form record is created, the form label should be updated with the form name desired for display, eg, “Demographics”, and the OID will remain as the abbreviated domain, ie, DM (Image 1).
Labels and Notes: A label is the prompt, also called the question text, and is displayed in the eCRF. The labels were modified in some cases to opt for wording that would be more intuitive to the end user within the context of the eCRF user interface, rather than to fully match CDASH. For traceability of metadata to CDASH, the CDASH label text was copied into a “Notes” metadata column in the eCRF form template. The Notes metadata is not displayed to the eCRF end user, however if the wording in the “Notes” column is preferred, the eCRF designer can copy the cell contents to the corresponding “label” column in the eCRF form definition (Table 2).
Example XLSForm definition for Demographics depicting “Notes” column.
| Type | name | Notes | label | bind::oc:itemgroup |
|---|---|---|---|---|
| begin group | DM1 | Demographics | ||
| date | DM_BRTHDAT | What is the subject’s date of birth? | Birth Date | DM |
| calculate | AGECALC | DM | ||
| integer | DM_AGE | What is the subject’s age? | Age: | DM |
| text | DM_AGEU | What is the age unit used? | What is the age unit used? | DM |
| select_one SEX | DM_SEX | What is the sex of the subject? | Sex | DM |
| Select_one ETHNIC | DM_ETHNIC | Do you consider yourself Hispanic/Latino or not Hispanic/Latino? | Ethnicity | DM |
| Select_multiple RACE | DM_RACE | Which of the following five racial designations best describes you? (More than one choice is acceptable.) | Race | DM |
| text | DM_RACEOTH | What was the other race? | Specify Other Race | DM |
| end group | DM1 | |||
Form Logic
CDASH defines basic skip logic that can be implemented in the eCRF using relevant logic. For example, most forms start with a leading question asking if the procedure or event occurred where a “yes” or “no” answer is expected. The response is then used in logic to show or to hide following form items. Relevant logic was also used to show or to hide items based on the selection of “Other”. Implementation of the logic expressions in the eCRF was carried out manually, based on interpretation of the requirements in CDASH.
The eCRF model also supports logic for edit checks and calculations. However, the CDASH literature suggests that these types of logics should be implemented on a study-specific basis.3 They have therefore not been included in the implementation.
Dissemination
To date, 33 Foundational CDASH domains have been implemented into OpenClinica forms using the mapping model described above. These encompass all the domains that are available on the CDISC eCRF Portal. Work is ongoing to cover additional CDASH domains to support therapeutic areas, such as Crohn’s Disease, which includes 20 disease-specific forms.5
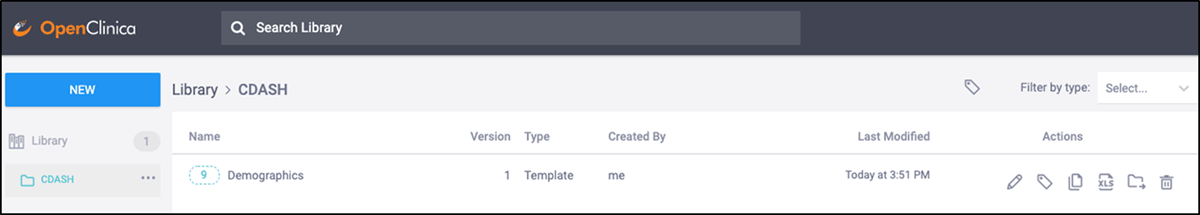
The EDC system used in the demonstration supports dissemination of the forms by making them available in the content library of each organization using the system, in a folder called ‘CDASH’. The CDASH forms can now be implemented for a study by “dragging and dropping” the form from the form library into a study (Images 2 and 3).
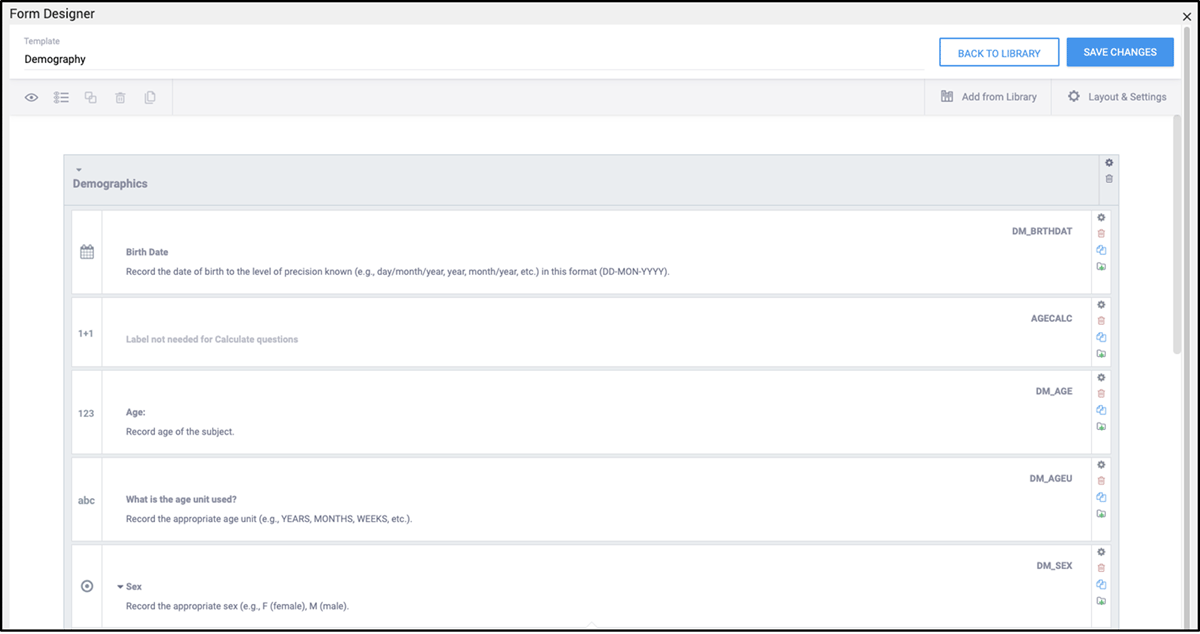
Once CDASH form definitions were in the form library, they were available for use in studies within the EDC system. The EDC supports editing the CRF within the UI-based Form Designer, or by downloading and editing the form definition using the Excel Template (Image 4).
An entire form can be added to a study and can be updated to reflect the given protocol requirements without disrupting the original library template. If there are study-specific forms that might pertain to other studies, they can also be uploaded to the library, via downloading their form template and then uploading it within Library Management.
Using a library of pre-made forms can help to streamline the process of building a study by cutting down the time taken in form definition and testing. With a properly managed form library each form only needs to be tested once to make sure it works for the general use case of the organization; it can then be regarded as validated for future studies. The form library allows for changes to be made either within the UI-based Form Designer, or it can be downloaded in XLS format to be edited (Images 4 and 5). Once used in subsequent studies, only the changes need to be tested.
Discussion
The main use cases that have been identified for pre-built CDASH forms are to aid rapid study start-up, to establish organizational eCRF standards, and to increase consistency. Reducing the need to translate protocols to eCRF specifications for commonly used forms takes a burden away from data managers and gives them more bandwidth to focus on study-specific forms. In terms of generating internal standards, presenting pre-made CDASH forms supports data element definitions and naming conventions that are familiar to, and can be easily accepted by, biostatisticians for data extraction, transformation, and analysis. Having standards may help to reduce the need for avoidable amendments to eCRFs and shorten overall study build time.
Future investigations should include assessment of user experience adopting and implementing CDASH forms from the OpenClinica form library. Collecting quantitative and qualitative feedback would help to the degree to which the benefits listed above are realized and may inform future enhancements to the OpenClinica system.7
Conclusion
A library of CDASH-based forms offers quick access to ready-made content for EDC study designers that removes barriers to the re-use of high-quality, expert-defined eCRFs. It creates opportunities to increase study efficiency, data consistency, and quality. With core content design standardized, greater focus can be placed on study-specific forms. Benefits include:
Greater alignment between data points that are captured in eCRFs and those expected by statisticians and scientists. This can mean fewer delays in design and fewer protocol amendments.
Faster implementation times and lower study start-up costs, including reduced testing and validation times.
Greater re-usability of data because of widely adopted naming conventions, data structures, and controlled terminologies.
eCRF content that represents expert consensus and is easy to customize to the needs of each research program/trial.
Acknowledgements
This project could not have been completed without the organization of the project by John Owen and Rhonda Facile. They both brought the CDISC team to assist with testing and design of the eCRFs and kept us all on the timeline for deliverables by making sure we were all actively engaged with this project.
We would also like to give a special thanks to the CDISC team, Richard Marshall, the Consultant Standards Developer, and Kathleen Mellars, the SDTM Consultant, who have brought their expertise and knowledge to the project. Without them, we would not have been able to bring this project to completion with the proper compliance to the metadata.
Competing Interests
The authors have no competing interests to declare.
References
1. CDISC.CDASH. Accessed February 22, 2022. https://www.cdisc.org/standards/foundational/cdash.
2. OpenClinica. Clinical Data Management, EPRO and ECRF. Published January 26, 2022. Accessed February 10, 2022. https://www.openclinica.com.
3. Gaddale JR. Clinical Data Acquisition Standards Harmonization importance and benefits in clinical data management. Perspect Clin Res. 2015 Oct–Dec; 6(4): 179–83. PMID: 26623387; PMCID: PMC4640009. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4640009/. DOI: http://doi.org/10.4103/2229-3485.167101
4. CDISC. https://www.cdisc.org/standards/data-exchange/odm.
5. CDISC. ECRF Portal. Accessed February 13, 2022. https://www.cdisc.org/kb/ecrf.
6. XLSForm.org. What is an XLSForm. Accessed February 13, 2022. https://xlsform.org/.
7. CDISC. SDTM and CDASH: Why you need both. Accessed February 12, 2022. https://www.cdisc.org/kb/articles/sdtm-and-cdash-why-you-need-both.
8. US Food & Drug Administration. What Are the Different Types of Clinical Research. Published January 4, 2018. Accessed February 13, 2022. https://www.fda.gov/patients/clinical-trials-what-patients-need-know/what-are-different-types-clinical-research.