Introduction
The World Medical Association’s Declaration of Helsinki (DoH) establishes principles for the ethical conduct of medical research. DoH revisions in 2013 added requirements to register human subjects research studies, asserted “the duty (of researchers) to make publicly available the results of their research on human subjects,” and recommended that doing so be a prerequisite for publication.1 Four years later, the International Committee of Medical Journal Editors (ICMJE) announced its decision to require that interventional clinical trials share individual participant data (IPD) sharing plans before participant enrollment in order to be eligible for publication in its member journals. This was based on the assertion that sharing IPD will “maximize the knowledge gained from the efforts and sacrifices of clinical trial participants.”2 The announcement does not explicitly define IPD, but the original policy proposal notes that it is data “underlying the results presented in the article (including tables, figures, and appendices or supplementary material).”3
Per ICMJE, the IPD sharing statements are required with the trial’s initial registration in ClinicalTrials.gov or other publicly accessible registry. They should include information regarding what data will be shared, whether other related documents will be available, and when and to whom the data will be available. ICMJE did not mandate sharing IPD, so it is currently acceptable to have a plan to not share any data. Changes to the IPD sharing statements can be made, and “should be…updated in the registry record.”4
With this study we aimed to gain insight into the following questions: Are researchers complying with the ICMJE policy? How are researchers responding to the Plan to Share IPD prompt in ClinicalTrials.gov? Are there any particular study characteristics that are associated with those who plan to share the IPD? To accomplish this, we analyzed the contents of the IPD plans that researchers at the University of Michigan (U-M) submitted with trial registrations for the first 27 months that the 2019 requirement was in effect.
Background
IPD plans are just one requirement within a larger landscape of research data sharing. Sharing research data upon completion of a study is a meaningful component of good stewardship practices.5 Redundant research effort due to inefficient knowledge sharing wastes time and resources and slows progress toward urgently needed discoveries.6 Compared with the pace of previous centuries of scientific research, the incredible speed and efficiency of the development of treatments and vaccinations related to the COVID-19 pandemic shows that the collaboration of the scientific community through data sharing can have a massively positive impact, as noted by the U.S. Office of Science and Technology Policy.7
The COVID-19 pandemic resulted in a surge of interest in research data sharing. This is reflected in a higher rate of declared intentions to share IPD among COVID studies in the first 18 months of the pandemic (up to 57%) than what was seen in previous reviews.8 However, a significant number of responses to questions regarding intent to share data were inadequate or contradictory.9 Even with a shared understanding of the value and necessity of data sharing for scientific progress, researchers’ demonstrated understanding of what is required of them appears to lag behind their stated willingness to share their data.
Data sharing requirements place a considerable burden upon researchers who must keep themselves apprised of new or changing policies, determine which apply to their particular situation, and then do their best to comply. Even researchers with good intentions and an earnest desire to contribute to the public and scientific communities, find this challenging. Research studies frequently have lean staffing and tight deadlines. As a result, some aspects of study design, planning, and implementation that have not been required by federal regulations, such as data sharing, have been understandably deprioritized.10
Additionally, many researchers are concerned that sharing data could be detrimental to their career. The data they gather forms the basis of their careers in the field, so immediately and freely sharing that data with others who might either misuse it, or use it to reach the next logical goal of the research ahead of them, holds little appeal.11
Ohmann, et al. conducted a review of studies published between 2001 and 2020, which suggested that the researchers involved in most studies were theoretically willing to share their data, as indicated via surveys, but this did not translate to a high rate of actual data sharing. The review noted that “[s]ome investigators may be reluctant to share their data, other[s] may simply not know how to proceed.”12 Additionally, Danchev et al. investigated declared versus actual data availability in three ICMJE journals (JAMA, Lancet, and New England Journal of Medicine) and found that “[o]nly 2 of 334 IPD sets were actually deidentified and publicly available,” noting that “a wide gap between declared and actual data sharing exists.”13
Because of the relative newness of the ICMJE requirement, research that examines, compares, or evaluates IPD plans within ClinicalTrials.gov is just beginning. Statham, et al. conducted the first review of IPD plans, with the goal of “establish[ing] baseline characteristics for the frequency and quality of these statements.”14 Their review of studies posted in the first six months of 2018, prior to the policy going into effect, revealed that at that time, only 5.5% of these registrations indicated that the researchers intended to share their IPD.14
The reviews by Ohmann, et al. and Statham, et al. also observed a pattern of inadequate understanding of how to construct a data or IPD sharing statement, based on the free-text responses available for analysis.12,14 This could be due to: unclear or varied requirements from disparate organizations, researchers not wanting to predict how they will share data in the future, or a relative lack of enforcement of requirements. This research suggests that there is significant opportunity to improve on data sharing standards and enforcement. Our study builds on this work by examining the details of IPD plans posted in ClinicalTrials.gov after the ICMJE policy was in place.
Setting
ClinicalTrials.gov provides public access to information about clinical studies. Clinical trials are required to register on ClinicalTrials.gov under the Food and Drug Administration (FDA) Amendments Act of 2007 (FDAAA), and under National Institutes of Health (NIH) policy since 2017.15,16 Information about trials is submitted and maintained by the sponsor or principal investigator (PI) of each study.
In December 2015, ClinicalTrials.gov added fields for recording information about IPD sharing.17 In June 2017 following the ICMJE announcement, a separate module was created in the trial registration form with six optional fields for capturing information about data sharing.17,18 Together these fields make up a trial’s Individual Participant Data Sharing Statement or Plan (IPD plan). The first field is a drop-down box labeled Plan to Share IPD, which registrants can bypass or answer “Undecided,” “Yes,” or “No.” To comply with ICMJE policy, trials beginning in 2019 must select either “Yes” or “No.”
The Definitions link in ClinicalTrials.gov provides brief guidance about the meaning of the fields and possible approaches to answers related to absolute or relative time frames. Minimal guidance is immediately visible within the registration interface, as shown in Appendix A.
The University of Michigan is a large public research university with a medical school and teaching hospital. In fiscal year 2021, the Medical School was awarded over 700 million dollars in sponsored awards and had over 2,495 active clinical trials.19 In June 2021, there were over 1,420 records in U-M’s two ClinicalTrials.gov accounts.
Most U-M PIs act as the Responsible Party, which means that only they can release their records to ClinicalTrials.gov. However, for those trials run through the Rogel Cancer Center, PIs review initial registrations and substantive updates but the Cancer Center’s ClinicalTrials.gov administrator can release the records. By law, a Responsible Party must verify the accuracy of their records annually until the trial is marked as completed and all legally required results are posted.
Methods
Using the administrator portal PRS (Protocol Registration and Results System) for ClinicalTrials.gov, a list of National Clinical Trial (NCT) numbers was retrieved for registered clinical trials in both U-M accounts: the Rogel Cancer Center, and the University of Michigan overall including the Medical School. Included trials were those with a start date of 1 January 2019 through those that were registered by 31 March 2021. The list of NCT numbers was then used to download current clinical trial registration data through the ClinicalTrials.gov API (Application Programming Interface) on 23 April 2021.
Data retrieved included the contents of the IPD sharing fields, along with the trial characteristics of interest: type of study (observational or interventional), anticipated or actual enrollment size, planned start date, anticipated or actual study completion date, list of collaborators, and if the trial tested an FDA regulated drug or device.
The original data pull included 243 studies. One trial was not a U-M registered trial but included a reference to one of the NCT numbers in the list. The ICMJE policy applies only to Interventional trials, so 31 Observational studies were removed. Thirteen “Withdrawn” trials that had “0” entered for their enrollment were also removed. The final data set had 198 analyzable trials.
The study team created a data collection form using Qualtrics (https://www.qualtrics.com) to sort and analyze the raw data according to the characteristics of interest. The methods section of Statham, et al. was consulted as a starting place for how to group the trial characteristics.14 IPD plan fields were first noted as either blank or having a response. If a response was provided, additional information about the responses to each of the IPD fields was captured. Appendix B lists the data collection form fields and options.
Each trial was coded by at least two authors; discrepancies between the coders’ classifications were resolved through discussion and rechecking the raw data. Descriptive statistics were then used to highlight rates of ICMJE compliance for each characteristic of interest, as well as responses to specific questions of the IPD sharing plan form.
Results
Table 1 shows selected characteristics of the 198 trials analyzed. Most trials were not FDA regulated (61%), and most trials were not NIH funded (66%). Additionally, most of the trials lasted less than 3 years (68%). Nearly half were small, with 50 or fewer participants either expected to enroll or actually enrolled in completed trials (48%).
Selected characteristics of the trials included in this study (n = 198).
| FDA regulated | ||
| Yes | 78 | 39% |
| No | 120 | 61% |
| NIH funded | ||
| Yes | 68 | 34% |
| No | 130 | 66% |
| Length of study | ||
| Less than 1 year | 38 | 19% |
| 1 year–less than 3 years | 96 | 49% |
| 3 years–less than 10 years | 64 | 32% |
| Enrollment | ||
| Small (1–50) | 95 | 48% |
| Medium (51–100) | 48 | 24% |
| Large (101–500) | 41 | 21% |
| Extra Large (501+) | 14 | 7% |
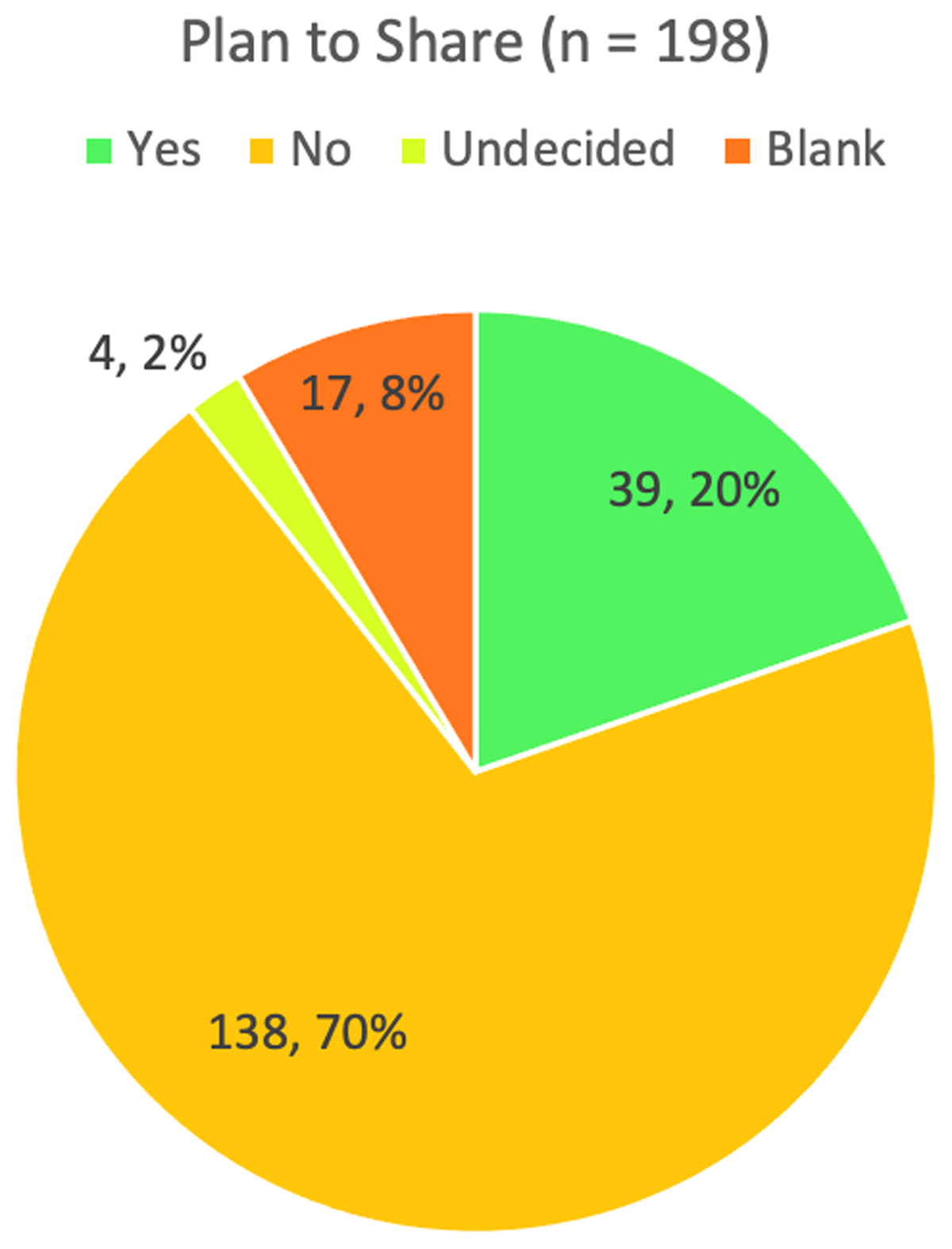
Figure 1 shows the responses to the Plan to Share field. More than two-thirds of trial registrations (138, 70%) answered “No,” they would not share IPD, while an additional 21 (10%) left the question as blank or answered “Undecided.” Only 39 trial registrations (20%) identified plans to share IPD.
Table 2 provides a breakdown of the Plan to Share responses by trial characteristics. Regarding FDA regulated status, the largest category was trials that are not FDA regulated that did not plan to share IPD (84 out of 198). Less than 25% answered “Yes” to IPD sharing, regardless of FDA regulated status. Most of the trials were not funded by NIH and did not plan to share IPD (100 out of 198). But among NIH funded trials, 37% said “Yes” compared with only 11% of non-NIH funded trials. There does not appear to be a strong connection between the size of the trial and the choice to share IPD or not, but in no enrollment size category did even 30% say that they would share IPD. A higher percentage of trials with medium and extra-large enrollment sizes indicated that they would share IPD, 27% and 29% respectively. Proportionally more studies that planned to collect data for one year or longer answered “Yes” to IPD sharing than those of shorter duration, 25% (1 year – less than 3 years) and 20% (3 years – less than 10 years) compared with 11% (less than 1 year). The only study characteristic for which “Yes” responses occurred more than 30% of the time was NIH funded trials.
Plan to Share responses and trial characteristics.
| Plan to Share | ||||||||
| Yes | No | Blank | Undecided | |||||
| FDA regulated | ||||||||
| Yes (n = 78) | 18 | 23% | 54 | 69% | 5 | 6% | 1 | 1% |
| No (n = 120) | 21 | 18% | 84 | 70% | 12 | 10% | 3 | 3% |
| NIH funded | ||||||||
| Yes (n = 68) | 25 | 37% | 38 | 56% | 3 | 4% | 2 | 3% |
| No (n = 130) | 14 | 11% | 100 | 77% | 14 | 11% | 2 | 2% |
| Enrollment | ||||||||
| Small (n = 95) | 16 | 17% | 70 | 74% | 7 | 7% | 2 | 2% |
| Medium (n = 48) | 13 | 27% | 32 | 67% | 3 | 6% | 0 | 0% |
| Large (n = 41) | 6 | 15% | 30 | 73% | 5 | 12% | 0 | 0% |
| Extra Large (n = 14) | 4 | 29% | 6 | 43% | 2 | 14% | 2 | 14% |
| Length of study | ||||||||
| Less than 1 year (n = 38) | 4 | 11% | 28 | 74% | 5 | 13% | 1 | 3% |
| 1 to less than 3 years (n = 96) | 19 | 20% | 72 | 75% | 5 | 5% | 0 | 0% |
| 3+ years (n = 64) | 16 | 25% | 38 | 59% | 7 | 11% | 3 | 5% |
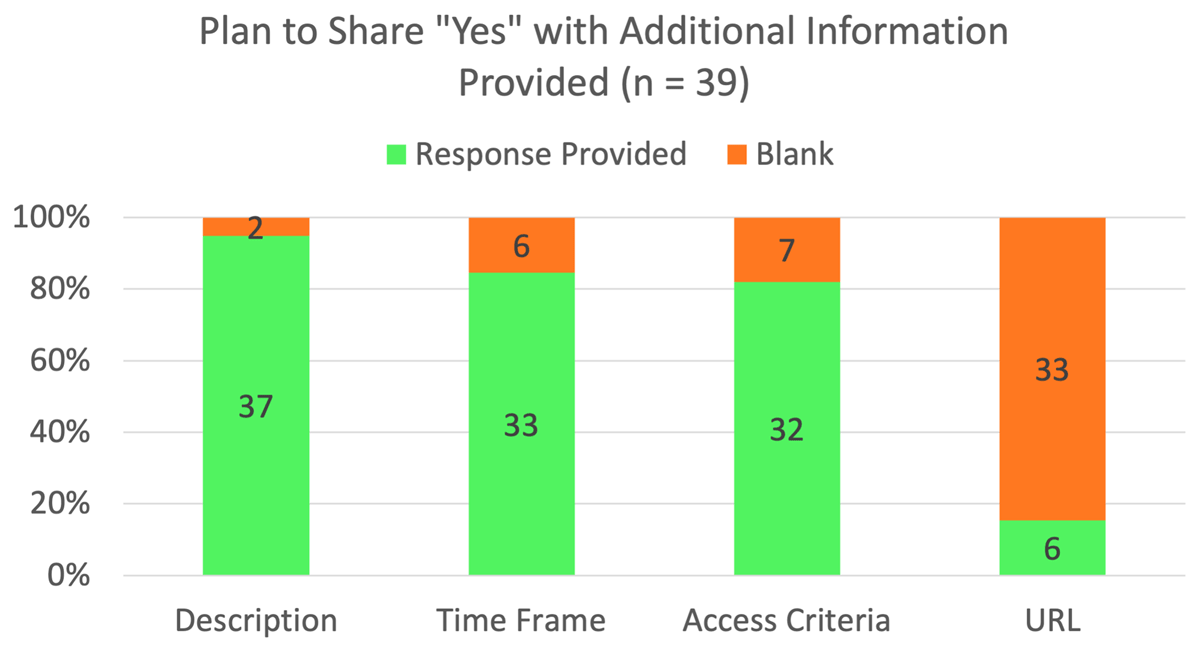
As Figure 2 indicates, most of the trials that indicated they would share IPD provided additional information in the other fields in the form. Specifically, 95% provided information in the Plan Description field, 85% provided information in the Time Frame field, and 82% provided information in the Access Criteria field. Six of the 39 trials (15%) provided a URL at the time of registration. For those trials that indicated a plan to share IPD, almost all provided information in the second field in the registration form, Plan Description. Two left this field blank, but 64% provided at least a minimal description of the data to be shared. The other 31% provided information pertaining to data sharing but did not provide an adequate description of the data to be shared.
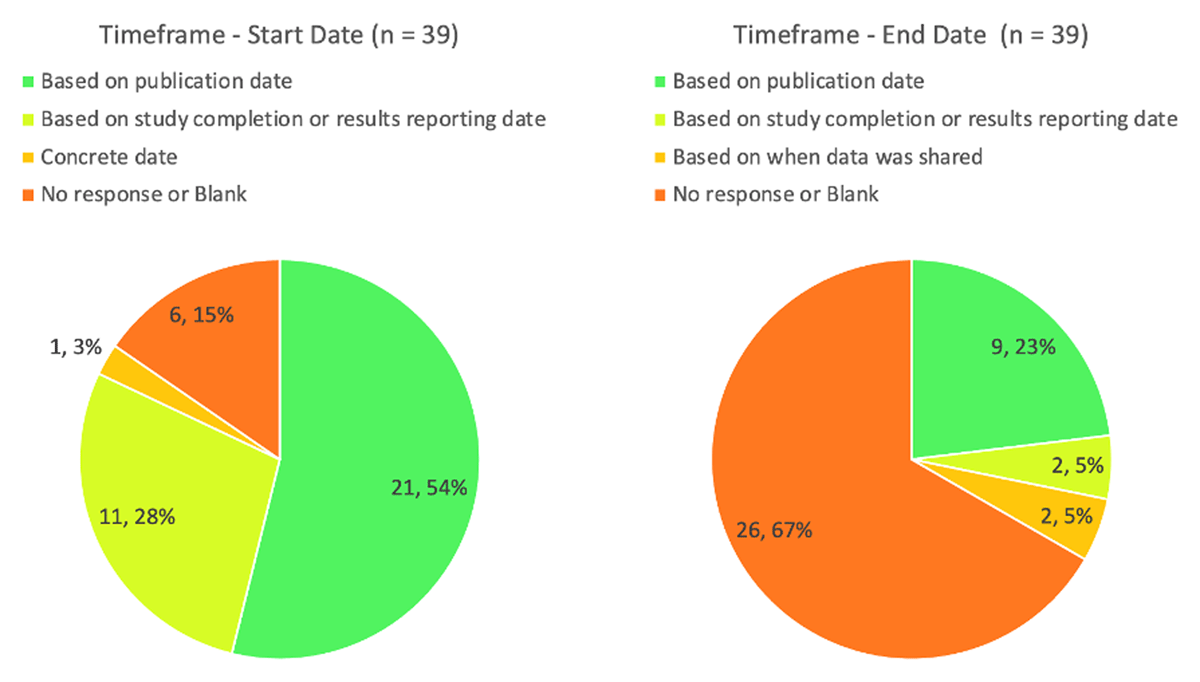
The Time Frame field should include both a start date and either a duration or end date. For those planning to share IPD, Figure 3 shows how start dates and end dates were described. Six left this field blank, even though they indicated that they were planning to share IPD. Just over half (21 of 39) tied the start date to a publication date, whereas just over a quarter based it on a study completion or results reporting date. Of those that planned to share IPD, two-thirds (26) did not include a duration or end date. Nine responses set an end date based on publication date, two based it on when the study completed or results were reported, and two based it on when data was shared.
In the Access Criteria field, 32 trials (82%) provided a response. ClinicalTrials.gov does not provide any help text for this field, but the ICMJE specified that the response should describe three items: with whom the data will be shared, for what types of analyses, and by what mechanism.2 While a preponderance of those responses (91%) provided some sort of mechanism for sharing, fewer specified with whom the data can be shared (78%), and only 59% provided information for what types of analyses. Regarding mechanisms, 47% noted that the IPD would be available via a repository or other open website, 31% noted that it would be available upon request to the PI, and the other 13% indicated some other way of making the data available (such as via a publication supplement or a combination of methods). Three of the trials (9%) that provided information in the Access Criteria field did not indicate a sharing mechanism. Table 3 shows the breakdown of responses in the Access Criteria field.
Access Criteria responses (n = 32; 7 blanks were not analyzed with these questions).
| Specifies with whom the data can be shared | ||
| Yes | 14 | 44% |
| No | 7 | 22% |
| Sort of – refers to selected repository’s policies | 11 | 34% |
| Specifies for what types of analyses | ||
| Yes | 10 | 31% |
| No | 13 | 41% |
| Sort of – refers to selected repository’s policies | 9 | 28% |
| Specifies by what mechanism | ||
| Yes – Available upon request to PI | 10 | 31% |
| Yes – Repository or other open website | 15 | 47% |
| Yes – Other | 4 | 13% |
| No | 3 | 9% |
Not all of those indicating that they would use a data repository specified which repository they would use. For those that would share via a repository, some either implied or specifically noted that the repository’s policies would determine with whom the data would be shared (11 of 15) and for what types of analyses (9 of 15).
Of the 39 trials that planned to share their data, six (15%) provided a response to the URL field in the form. Two of those six responses contained broken links, two routed to a repository‘s website, and two went to a website that was related to the research project.
While coding the trials, the authors noted if there were any potential problems with the IPD sharing plan, such as conflicting or confusing access criteria, missing or ambiguous time frame information, the implication of the use of a repository without explicitly stating that as the plan, or minimal or no provided details. Of those that said they planned to share IPD, only 23% had no potential problems. The rest, a total of 30 trials out of 39, had at least one potential problem noted by an author.
Discussion
At a minimum, the ICMJE policy requires an answer to the question of whether the researcher plans to share IPD but accepts “No” as a valid answer. It is encouraging that the vast majority (90%) of trials in this study have provided an acceptable answer in line with the ICMJE policy. This suggests that the policy is driving behavior, as most researchers are complying. However, there is room for improvement; additional efforts from ICMJE, ClinicalTrials.gov, and institutions could help ensure that all researchers provide an acceptable response to at least the first question in this section of the trial registration.
In this study, 20% of researchers stated that they planned to share data. This is an increase over the Statham, et al. findings of 5.5% indicating in 2018 that they would share IPD.14 However, not all of those 20% provided clear and complete sharing plan descriptions within their ClinicalTrials.gov records. Enormous opportunity remains to realize the ICMJE goal of having the “sharing of deidentified individual participant data [become] the norm.”2 Future research could apply this study’s methodology to more than one institution to assess the broader landscape of IPD sharing plans and to track the evolution of these plans over time.
Additional education and resources from ICMJE could help researchers to provide more useful responses in each of the IPD sharing statement fields. Some researchers put information into the Plan Description field that more properly should have been in the Access Criteria field. Some did not fully describe the data to be shared. Many did not provide an adequate response in the Time Frame field for how long the data would be available. Some of the information in the Access Criteria field was implied or hinted at, rather than being explicitly written out. For example, the statement “The data will be made freely available after any personally identifiable information is removed” hints that a repository may be used but no other part of the plan identified one. If a data repository is referred to in the plan, the ICMJE should clarify if the plan needs to reiterate the repository’s various access or preservation policies and procedures, or if an accurate URL reference is sufficient to indicate the access and preservation information.
In addition to the ICMJE providing more resources, bolstering the IPD plan section of the ClinicalTrials.gov registration form may encourage more accurate, complete, and internally consistent responses. User experience research could help to determine if more useful field labels could be established, if requiring certain fields to be completed would improve IPD plans, or if there is a more effective way to display the notes, help text, or examples for each of the fields. For example, it might be more intuitive for users if the Plan Description field were re-named Data Description to better specify what information should be there. As ClinicalTrials.gov is in the midst of a multi-year modernization project to make its system more intuitive and user-friendly, this could be an optimal time for such changes.20
Recommendations for time frames in IPD sharing plans should be developed to improve plan clarity. The examples from ICMJE use a publication date as an anchor for the time frame (eg, “Immediately following publication”2), but as it is possible for multiple publications to result from one trial, such a reference may obfuscate rather than illuminate the intended timeframe. It may be more practical to use study completion or results reporting dates as anchors for the time frame, as those are established dates that appear within ClinicalTrials.gov.
The landscape of data sharing continues to change. The NIH’s new Data Management and Sharing Policy is pushing researchers and data repositories toward more openness.21 As more biomedical researchers are required to write data sharing plans, they will become increasingly practiced in making data sharing decisions and in describing and sharing their data. A data management and sharing plan written at the time of an NIH application can easily translate into an IPD sharing plan when registering a trial. Additionally, more researchers will gain experience sharing data via a data repository since it is encouraged in the new NIH policy. As data sharing practices evolve, researchers will benefit from their institutions and funding agencies making additional investments in training opportunities, infrastructure, and incentives that support data management and data sharing activities. Any investments made will benefit from the expertise of data librarians, informationists, data scientists, data managers, data curators, data storage specialists, human subjects protection officers, and regulatory specialists. Future research could track the effectiveness of these efforts in improving the quality and type of information provided in IPD plans.
Another area for additional research is examining how these plans are assessed by ICMJE editors. Although ICMJE editors have adopted this policy, whether and to what degree they are checking the adequacy of IPD plans is not fully understood or explored.
Limitations
Our study has limitations. We only looked at studies from a single institution. We did not capture information about collaborations with other institutions which could be a useful study characteristic to consider since institutional data sharing policies could impact an IPD plan. This study captures a snapshot of what IPD sharing plans looked like at one point in time, without analyzing any changes to the records in ClinicalTrials.gov over the course of the trials.
Conclusions
There are three components to normalizing the practice of widespread data sharing. First, researchers must consider data sharing at the planning stage of a research project. 90% of trials in this study show that U-M researchers have done that, at least minimally. Second, researchers must be motivated to share data. Only 20% of the trials we analyzed noted that they were planning to share their data. Third, researchers will need to follow through on implementing the data sharing plans that they submit. This study did not delve into implementation of plans, but the influence of the ICMJE policy appears to be a step in the right direction. The ongoing emphasis on sharing data, which will ultimately improve IPD sharing statements, will require the continued engagement of researchers, data experts, research support offices at institutions, funders, the ICMJE, and ClinicalTrials.gov. Improving IPD sharing statements supports the broader effort to make scientific data more accessible and more completely honors the contributions of the trial participants.
Appendix
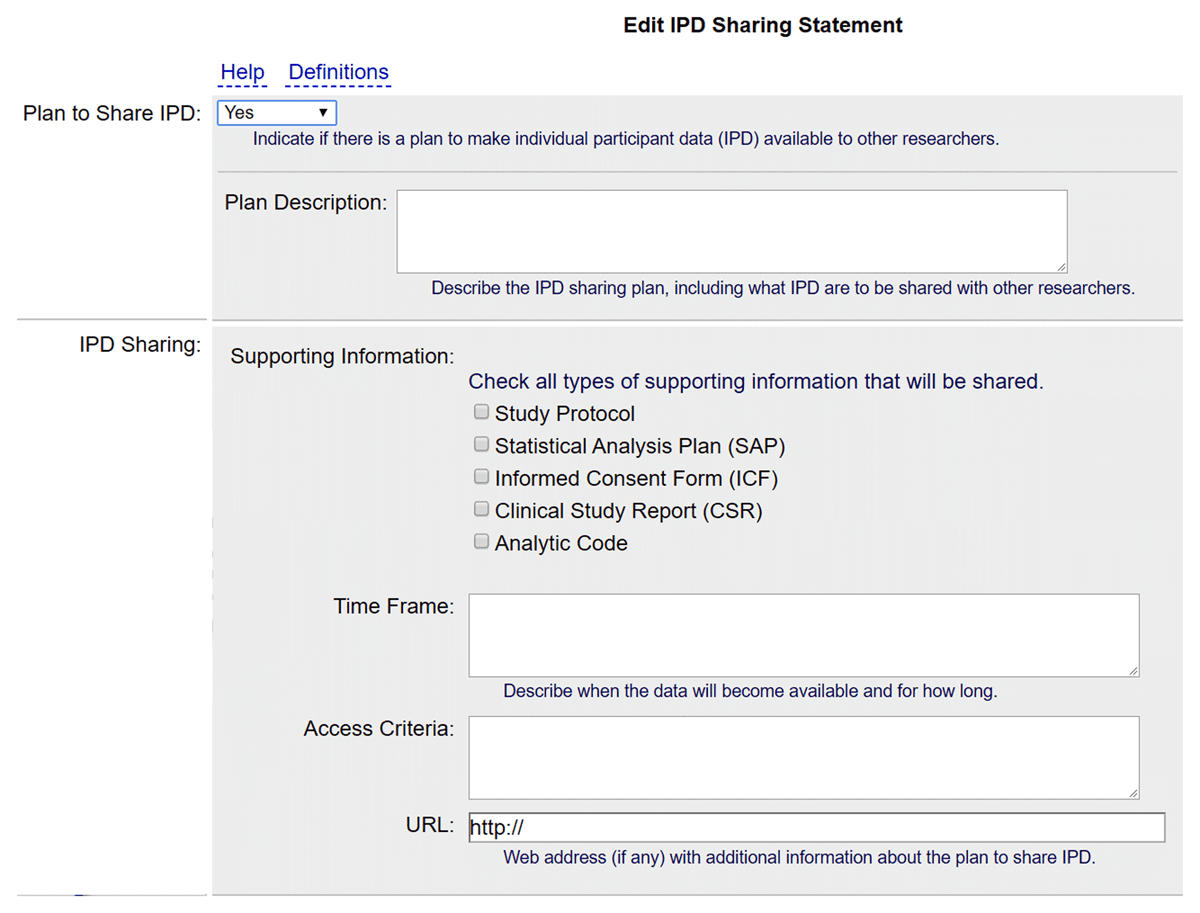
Screenshot of the IPD Sharing Statement module of the ClinicalTrials.gov registration form, showing all fields and notes. Captured August 1, 2022.
Data collection form fields and options.
| Study characteristics | |
| Study ID (NCT number) | Open text field |
| Study type | • Interventional • Observational |
| Study is FDA regulated | • Yes • No |
| Collaborates with NIH funder | • Yes • No |
| Length of trial (based on Start Date and Completion Date) | • Less than 1 year • 1 year – less than 3 years • 3 years – less than 10 years • 10 years or more* |
| Enrollment count | • Small (1 – 50) • Medium (51 – 100) • Large (101 – 500) • Extra Large (501+) |
| IPD Sharing Plan | |
| Plan to share | • Yes • No • Undecided • Blank |
| Description |
|
| Additional information (Select all that apply)** | • Analytic Code • Clinical Study Report • Informed Consent Form • Statistical Analysis Plan • Study Protocol • None Selected |
| Time frame |
|
| Access criteria |
|
| URL |
|
| Potential problems in IPD plan (select all that apply) | • Left "Undecided" or blank • Conflicting or confusing access information • Information about the plan is provided but not in appropriate boxes • Uses the exact same wording as ICMJE examples table (from 2017 publication) • Other [fill in details] ____________________ • No obvious issues |
-
* No trials in our final data set had a time frame of 10 years or more.
** Although the Additional Information field was included in the data the study team did not analyze this field.
Data Availability Statement
The data for this study is publicly available. Samuel SM, Wilson DL, Fleming E. 2023. Data for Evaluating Individual Participant Data Plans for ICMJE Compliance: A Case Study at University of Michigan [Data set], University of Michigan – Deep Blue Data. https://doi.org/10.7302/72tg-ap24.
Acknowledgements
Special thanks to staff at the National Institutes of Health who met with us to confirm that we were using the ClinicalTrials.gov API correctly. Additional thanks to our colleagues Marisa Conte, Tyler Nix, and Cathy Spino for reviewing the manuscript and providing feedback.
Competing Interests
The authors have no competing interests to declare.
References
1. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013; 310(20): 2191. DOI: http://doi.org/10.1001/jama.2013.281053
2. Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials — A requirement of the International Committee of Medical Journal Editors. N Engl J Med. 2017; 376(23): 2277–2279. DOI: http://doi.org/10.1056/NEJMe1705439
3. Taichman DB, Backus J, Baethge C, et al. Editorial – Sharing clinical trial data: A proposal from the International Committee of Medical Journal Editors. Ethiop J Health Sci. 2016; 26(1): 2. DOI: http://doi.org/10.4314/ejhs.v26i1.2
4. ICMJE|Recommendations|Clinical Trials. Accessed December 9, 2022. https://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html#one
5. Kalkman S, Mostert M, Gerlinger C, van Delden JJM, van Thiel GJMW. Responsible data sharing in international health research: a systematic review of principles and norms. BMC Medical Ethics. 2019; 20(1): 21. DOI: http://doi.org/10.1186/s12910-019-0359-9
6. Resnik DB. Stewardship of research resources. Accountability in Research. 2019; 26(3): 246–251. DOI: http://doi.org/10.1080/08989621.2019.1585819
7. White House Office of Science and Technology Policy (OSTP). Ensuring Free, Immediate, and Equitable Access to Federally Funded Research. Executive Office of the President of the United States; 2022. Accessed December 9, 2022. https://www.whitehouse.gov/wp-content/uploads/2022/08/08-2022-OSTP-Public-Access-Memo.pdf. DOI: http://doi.org/10.1016/j.cct.2022.106709
8. Larson K, Sim I, von Isenburg M, et al. COVID-19 interventional trials: Analysis of data sharing intentions during a time of pandemic. Contemporary Clinical Trials. 2022; 115: 106709. DOI: http://doi.org/10.1016/j.cct.2022.106709
9. Li R, von Isenburg M, Levenstein M, Neumann S, Wood J, Sim I. COVID-19 trials: declarations of data sharing intentions at trial registration and at publication. Trials. 2021; 22(1): 153. DOI: http://doi.org/10.1186/s13063-021-05104-z
10. van Panhuis WG, Paul P, Emerson C, et al. A systematic review of barriers to data sharing in public health. BMC Public Health. 2014; 14(1): 1144. DOI: http://doi.org/10.1186/1471-2458-14-1144
11. Cheah PY, Tangseefa D, Somsaman A, et al. Perceived benefits, harms, and views about how to share data responsibly: A qualitative study of experiences with and attitudes toward data sharing among research staff and community representatives in Thailand. Journal of Empirical Research on Human Research Ethics. 2015; 10(3): 278–289. DOI: http://doi.org/10.1177/1556264615592388
12. Ohmann C, Moher D, Siebert M, Motschall E, Naudet F. Status, use and impact of sharing individual participant data from clinical trials: a scoping review. BMJ Open. 2021; 11(8): e049228. DOI: http://doi.org/10.1136/bmjopen-2021-049228
13. Danchev V, Min Y, Borghi J, Baiocchi M, Ioannidis JPA. Evaluation of data sharing after implementation of the International Committee of Medical Journal Editors data sharing statement requirement. JAMA Network Open. 2021; 4(1): e2033972. DOI: http://doi.org/10.1001/jamanetworkopen.2020.33972
14. Statham EE, White SA, Sonwane B, Bierer BE. Primed to comply: Individual participant data sharing statements on . PLOS ONE. 2020; 15(2): e0226143. DOI: http://doi.org/10.1371/journal.pone.0226143
15. Requirements for registering & reporting NIH-funded clinical trials in ClinicalTrials.gov|grants.nih.gov. Accessed December 9, 2022. https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
16. FDAAA 801 and the Final Rule – ClinicalTrials.gov. Accessed December 9, 2022. https://clinicaltrials.gov/ct2/manage-recs/fdaaa
17. ClinicalTrials.gov Protocol registration and results system: What’s New. Accessed December 9, 2022. https://register.clinicaltrials.gov/prs/html/whats-new.html
18. Bergeris A, Tse T, Zarin DA. Trialists’ intent to share individual participant data as disclosed at . JAMA. 2018; 319(4): 406–408. DOI: http://doi.org/10.1001/jama.2017.20581
19. Facts & Figures. University of Michigan Medical School. Published October 13, 2016. Accessed August 3, 2022. https://medicine.umich.edu/medschool/about/facts-figures
20. ClinicalTrials.gov Modernization – ClinicalTrials.gov. Accessed August 4, 2022. https://clinicaltrials.gov/ct2/about-site/modernization
21. Jorgenson LA, Wolinetz CD, Collins FS. Incentivizing a New Culture of Data Stewardship: The NIH Policy for Data Management and Sharing. JAMA. 2021; 326(22): 2259–2260. DOI: http://doi.org/10.1001/jama.2021.20489